Update 46
UK news
Parents ripped off by potentially harmful 'toddler milks'
The baby milk industry has a cunning plan to boost profits and bypass restrictions on how it markets infant formula. We are seeing products for older babies with the same branding as the infant formula being marketed in the UK and around the world. Follow-on milks are marketed for use from 6 months and so-called Growing-Up Milks (GUMs) for children from one-year old.
The World Health Assembly said follow-on milks are unnecessary when they were first introduced – infant formula can be used if babies are not breastfed.
The UK National Health Service (NHS) Choices website says:
Follow-on milks are available for babies over six months but there is no need to change over to these. Cows' milk can be mixed with food from six months and whole cows' milk can be given as a drink from one year.
The UK consumer organisation Which? has highlighted the high sugar levels and said 'Parents could save more than £500 a year by giving their child cow’s milk instead of toddler milk.' See the Which? report.
In its expert analysis, First Steps Nutrition says:
Fortified milks are frequently high in sugar and are likely to contribute to higher energy intakes, which may contribute to chronic disease, and the voluntary fortification of foods and drinks needs to be questioned as there is increasing evidence that giving additional nutrients to those who do not need them may have adverse consequences.
Danone recruited the charity Tommy's to promote GUMs through its baby race in 2013, with claims that the ASA ruled in 2014 are misleading:
Even with a typical diet, it’s not easy for toddlers to get all the nutrients they need. Cow & Gate Growing Up Milk is a great way to help them get some of the hard-to-get nutrients into their diet: Just 2 x 150ml beakers a day help top up your toddler’s diet with Vitamin D for development of bones, omega 3 (an essential fatty acid) and iron for brain development.
These claims were promoted during a 'baby and toddler event' in ASDA supermarkets in February 2014 with GUMs sold at discount.

People were encouraged to put questions to ASDA pharmacy staff and to visit ASDA’s 'Baby and Toddler Club' website as a 'trusted resource for nutrition and feeding advice'.
Trusted?
The site has a disclaimer in small print saying the publishers do not 'make any representations as to the accuracy or efficacy of the information provided nor do they assume any responsibility for errors, omissions or contrary interpretation of the subject matter herein.'
NHS Choices, on the other hand, stands by its advice: 'Accuracy: NHS Choices content will be accurate, balanced and transparent. Information given will be based on the best available scientific evidence and data sources.'
International concerns
In 2011 the German Federal Institute for Risk Assessment found no advantage of growing-up milks compared to reduced fat cow milk and German Consumer Centres found they were 4 times as expensive as normal milk.
In 2012 the Australian Government's infant feeding guidelines said: 'Toddler milks and special and/or supplementary foods for toddlers are not required for healthy children.'
In 2013 the European Food Safety Authority concluded that GUMs have no additional value to a balanced diet.
Danone promotes its Cow & Gate brand by sponsoring Barnardos Ireland’s 'Big Toddle'.

Also see pg 31.
UK news
Tommee Tippee - perfect violation

Powdered formula is not sterile. It should be mixed with water above 70oC to kill any harmful bacteria that it may contain. The bottle then has to cool before feeding.
Tommee Tippee is marketing a machine that uses just a small volume of water to do this and then tops up with colder water. It says a bottle can be made ready in just two minutes.
Of course, it doesn't take an expensive machine to do this – people can do it in the home using a kettle and previously boiled or filtered water. But is it safe? WHO did not recommend this method, so we have asked for its views. An industry food safety expert has questioned how the hygiene of the closed mechanisms will be ensured.
Tommy Tippy promotes formula feeding as 'closer to nature' as in the special display in Toys R' Us, above, which is a perfect example of misleading marketing regardless of the safety issue.
UK news
Danone Cow & Gate advert banned

A television advertisement for Danone’s Cow & Gate follow-on milk was banned by the UK Advertising Standards Authority (ASA) in January 2014 as it misled viewers.
Such advertisements should not appear at all under international rules, but as these have not been implemented in the UK, we made use of the ASA system. We asked if the claims made in the advert had been approved by the European Food Safety Authority (EFSA) as required in the EU.
The ASA found that Danone had gone too far with its claims that 'Cow & Gate follow-on milk provides Calcium for strong bones', and 'Cow & Gate follow-on milk provides … iron for brain development'. Its ruling (A13-234819) states: 'consumers would not understand the adapted wording used in the ad to have the same meaning as the authorised wording.'
Our comments on the ruling were reported on the UK’s most visited news website, the Daily Mail (with a shorter article in the print edition):
Advertisements such as Danone's suggest that follow-on milks provide health benefits, but they are unnecessary products and Danone is simply ripping parents off, using false claims to make them think they need these milks. Yes, calcium is needed for normal growth and bone development in children and iron contributes to normal cognitive development, but these are provided by a normal diet and Danone’s products offer nothing special other than a way for the company to fill its pockets. The cost of these multi-million pound advertising campaigns goes onto the price of the milks making them even more expensive.
The article also reported our concerns that the ASA decision would have little impact:
We have won repeated cases proving that claims made for baby formula do not stand up to scrutiny, but the firms continue using them regardless because the ASA is a toothless body requiring no corrections and levying no fines.
Many of these practices also break the law in our view, but Trading Standards and the Department of Health are failing to hold companies to account. Companies should be prosecuted and fined for repeated violations.
UK formula regulation
Danone dismisses Department of Health rules
Last year, Baby Milk Action published the report Look What They’re Doing in the UK – 2013 showing how baby milk companies are breaking marketing rules in the UK. These are the Infant Formula and Follow-on Formula Regulations (2007) and the associated Guidance Notes.
Danone responded to our report by saying, 'we do not accept the Guidance Notes have the ability to "control" or "prohibit" certain practices.'
Danone dismisses the Guidance Notes because they say things like follow-on formula advertising must not: 'promote a range of formula products by making the brand the focus of the advert, rather than specific products (e.g. where specific products are mentioned only in a footnote or in a picture of a tin of formula within the advertisement).'
This is exactly what Danone did with its Cow & Gate advertisement on page 10. It showed a pack shot of follow-on milk, with a label virtually identical to that on the infant formula in the range.
 Danone is using the same strategy with its latest Aptamil Pronutra advertising. It also breaks the rule for labels. The Guidance Notes state: 'the specific terms "infant formula" and "follow-on formula" should be clearly featured on the packaging, in a font size no smaller than the brand name.' [Emphasis added.]
Danone is using the same strategy with its latest Aptamil Pronutra advertising. It also breaks the rule for labels. The Guidance Notes state: 'the specific terms "infant formula" and "follow-on formula" should be clearly featured on the packaging, in a font size no smaller than the brand name.' [Emphasis added.]
Look at the advertisements – these terms are lost alongside the branding.
The theme of Danone's latest mass media formula advertising campaign is 'The closer we look the more we discover.'
It says that it has spent '30 years studying breastmilk’ and has produced its ‘most advanced formula yet.'
It highlights the supposed benefits of ingredients for visual, brain and bone development.
Danone encourages people to search for information on Aptamil Pronutra on the Internet, which takes them to a website promoting the full range of formulas.
The Guidance Notes state, 'In order to achieve compliance, companies will therefore need to ensure that formula advertising does not: focus primarily on the promotion of ingredients, or the effect of ingredients, which are common to both follow-on formula and infant formula.'
The Guidance Notes were introduced by the Food Standards Agency in 2008 following widespread consultation on interpreting the law, including with the baby food industry. They became the responsibility of the Department of Health (DH) on 1 October 2010 with the change in the UK Government.
The DH is also responsible for authorising – or not – company materials for distribution in the health care system.
At the same time, however, DH promotes the Change4Life health promotion campaign. Corporate partners? Danone and Nestlé. Over 2,000 people have signed our petition on Change.org calling for an end to this unacceptable conflict of interest.
EU News
EFSA research renews calls for a ban on claims
 Glenis WillmottThe European Food Safety Authority (EFSA)1 published the preliminary work it will use for its forthcoming evaluation of essential formula ingredients. The review is part of the ongoing overhaul of all EU baby food legislation.
Glenis WillmottThe European Food Safety Authority (EFSA)1 published the preliminary work it will use for its forthcoming evaluation of essential formula ingredients. The review is part of the ongoing overhaul of all EU baby food legislation.
The findings have prompted renewed calls from socialist MEP Glenis Willmott (above) to ban health and nutrition claims on follow-on formulas. An extensive literature review found no scientific evidence, or insufficient evidence, to support the inclusion of many of the ingredients commonly used in formulas.
Speaking to EU Food Policy, Ms Willmott said:
'If there are no scientifically proven benefits then we should not be allowing advertising of follow-on formula and we certainly should not allow companies to use health claims which guilt trip parents into buying more expensive formula unnecessarily.'
In 2011 Ms Willmott led the call in the European Parliament to veto a claim that DHA improved eyesight:
Whilst I received the support of a majority of MEPs, it was not enough to stop them going ahead. With EFSA’s evidence now agreeing there is no benefit, the Commission must urgently revise its approach.
Together with the Baby Feeding Law Group we have been calling for an end to 'optional ingredients' because they open the door for claims that are both misleading and highly promotional, incorrectly suggesting that a formula could be better than breastfeeding. If an ingredient is essential it should be in all formulas.
The Commission is holding regular meetings with Member States to discuss the legislation. Under the new rules MEPs can attend as observers. EFSA will publish its final opinion in April and will hold a open consultation in June.
The External report for EFSA:
● Compared the effects of different additives (protein, probiotics, DHA/ARA, cholesterol, selenium iron etc) on growth and health;
● Compared various formulae with breastmilk;
● Assessed research as being moderately biased if fully or partially funded by industry, and severely biased if it had epidemiological flaws.
It concluded that there was:
● No evidence of any beneficial effect for different amounts of protein, DHA/ARA, prebiotics, probiotics, cholesterol, palm oil, selenium or nucleotides;
● No evidence of serious, general or specific nutrient deficiency in Europe;
● No need for general supplementation of iron, iodine, selenium etc;
● No evidence that formulae enriched with these nutrients would have any effect, except maybe in vulnerable subgroups.
We find the case for labelling infant formula or follow on formula with health or nutrition claims entirely unsupportable. If an ingredient is unequivocally beneficial as demonstrated by independent review of scientific data it would be unethical to withhold it for commercial reasons. Rather it should be made a required ingredient of infant formula in order to reduce existing risks associated with artificial feeding.
UK Scientific Advisory Committee on Nutrition 2007
1. Preparatory work for the evaluation of the essential composition of infant and follow-on formulae and growing-up milk. http://www.efsa.europa.eu/en/supporting/pub/551e.htm
New marketing strategies
Milks for mothers

 Malnutrition is a shameful world problem that must be tackled. It is also important that pregnant and lactating mothers have access to enough and appropriate food. But is the marketing of micronutrient powders and special formulas and supplements for mothers and babies the answer?
Malnutrition is a shameful world problem that must be tackled. It is also important that pregnant and lactating mothers have access to enough and appropriate food. But is the marketing of micronutrient powders and special formulas and supplements for mothers and babies the answer?
While Folic Acid, Vitamin D and other supplements may be important for pregnant women, requirements will vary from region to region and sector to sector. Nutrition interventions must be based on independent systematic reviews of evidence and must be properly controlled and managed by governments, not left to companies, many of whom will push anything as long as it is profitable.

When governments take action to ban the promotion of breastmilk substitutes, companies seek other ways to reach mothers. The promotion of formulas for mothers allows them to claim to be supporting breastfeeding while undermining womens' confidence in their bodies' competence to do it. When products are cross-branded with baby milks the door is opened to a range of products for the whole life cycle. Soon everyone starts to believe that unprocessed, cheaper and often more nutritious foods are somehow lacking.
It's not just the manufacturers of breastmilk substitutes. Companies such as Ajinomoto (the Japanese sweetener and MSG producer), DSM (Europe’s largest vitamin and formula ingredients manufacturer) and Pepsi are all seeing the commercial potential of the 'first 1000 Days.'

World Health Assembly Resolution 55.25 urges Member States:
to ensure that the introduction of micronutrient interventions and the marketing of nutritional supplements do not replace, or undermine support for the sustainable practice of, exclusive breastfeeding and optimal complementary feeding.
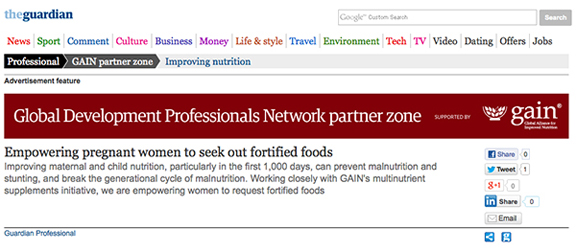
GAIN-sponsored ‘Nutrition Hub’ on the Guardian website. The headline: 'Empowering pregnant women to seek out fortified foods' uses the term 'empowering' instead of 'advertising to.' It suggests that fortified products are essential for pregant women. January/February 2014.
Infant feeding in emergencies
UN urges no formula donations after Philippines Typhoon
 Arugaan mothers
Arugaan mothers
 Arugaan with mothers
Arugaan with mothers
Arugaan volunteers help mothers
All infants with diarrhea, near death with dehydration and sent to hospitals straight from C-130 (planes) are all bottle - and formula-fed...All babies who are breastfed are well and not even sick despite getting soaked cold last Friday (when Yolanda hit).
Haide Acuna, a breastfeeding campaigner visiting the evacuation centers in Cebu 1
With our water and food supply running low, I just breastfed and breastfed my son...If our child had been dependent on milk formula, I would have taken part in looting, too....When we went out to forage for food, I saw people looting formula milk for their babies...Even if the baby were bottle-fed before Yolanda, the baby could be breastfed anytime.
Danika Christin Magoncia, a Yolanda survivor from Tacloban 1
Typhoons are common in the Philippines, but the one that hit Tacloban City and Cebu in November was one of the strongest ever recorded. People were left without food, water and power. In this context breastfeeding is a lifeline for babies.
In the aftermath over 20 UN agencies and NGOs issued an urgent appeal, highlighting the risk to life from formula donations. Donor agencies, NGOs, media and individuals were urged to avoid calls for and sending of baby feeding products into the area and called for needs assessments by qualified health and nutrition workers trained in infant feeding.
Industry threat to the Philippines
Meanwhile the baby food industry - under the guise of the Infant and Pediatric Nutrition Association of the Philippines (IPNAP) has been trying to weaken the existing protection of the Philippines Milk Code in order to legitimise many of its harmful practices ices, such as advertising of formulas, industry contact with mothers for so-called educational purposes, sponsorship and training health workers. Lactation breaks at work would also be unpaid. They also want to be able to give donations of baby milks during emergencies. WHO, UNICEF Philippines and the International Labour Office (ILO) are urging the Philippines Government to reconsider any relaxation of the Milk Code.
For more information follow these leads:
Operational Guidance on Infant and Young Child Feeding in Emergencies, v2.1, Feb 2007
www.ennonline.net/resources/6
IBFAN http://ibfan.org/infant-feeding-in-emergencies/125
http://www.ibfanasia.org/ife/unicef_media_joint-statement.pdf
A first hand account from Velvet Here: http://info.babymilkaction.org/velvet
Groups urge breast milk for babies in storm shelters, Inquirer News. goo.gl/sCffm7
Publications and research
What’s inappropriate promotion?
The Resolution passed at the 2012 World Health Assembly Resolution (WHA) called for 'clarification and guidance on the inappropriate marketing of foods for infants and young children'.
WHO convened a Working Group in June to explore this issue, and has arrived at 5 criteria that could be used to inform policies. We hope that these will be highlighted in a new resolution at the forthcoming WHA in May.
Promotion is inappropriate if:
● it undermines recommended breastfeeding practices;
● contributes to childhood obesity and noncommunicable diseases;
● the product does not make an appropriate contribution to infant and young child nutrition in the country;
● it undermines the use of suitable home-prepared and/or local foods;
● it is misleading, confusing, or could lead to inappropriate use.
See Annex 2, Page 10 on WHO's Report by the Secretariat on Maternal, infant and young child nutrition (EB134/15)


Research and news
Human milk protein neutralises HIV
In October 2013 the National Academy of Sciences published research from Duke University about a protein called Tenascin C in human breastmilk that neutralizes HIV and in most cases, prevents it from being passed from mother to child. Eventually, they say, the protein could potentially be valuable as an HIV-fighting tool for both infants and adults that are either HIV-positive or at risk of contracting the infection.
Discovered: A Natural Protein in Breast Milk That Fights HIV.
US child obesity levels decline
Obesity data from the US Centre for Disease Control (CDC) published in the February 26 issue of the Journal of the American Medical Association, show a 43% decline in obesity among children aged 2 to 5 years. From nearly 14 percent in 2003-2004 to just over 8 percent in 2011-2012 – based on CDC’s National Health and Nutrition Examination Survey (NHANES) data. CDC speculates that this could be due to decreased consumption of sugary drinks and increased breastfeeding rates in the United States.
Pope Francis blesses breastfeeding

There was much media coverage in January about when Pope Francis told the mothers of babies’ he was baptising: 'If they are hungry, mothers, feed them—without thinking twice—because they are the most important people here.'
See: Lancet Global Health Blog by Dr Chessa Lutter, Regional Advisor on Food and Nutrition, Pan American Health Organization (PAHO).
Cochrane Review finds no evidence for Ready-to-Use
 IBFAN is calling for a review of UN and NGO programmes following the publication of two systematic reviews by the Cochrane Collaboration - an international benchmark for evaluating health interventions. Cochrane could not find evidence that commercial Ready-to-Use Foods and lipid-based supplements were any better than flour porridge made locally from enriched blended food for the treatment of severe and moderate acute malnutrition. Ready-to-Use products are often used in appeals (see right).
IBFAN is calling for a review of UN and NGO programmes following the publication of two systematic reviews by the Cochrane Collaboration - an international benchmark for evaluating health interventions. Cochrane could not find evidence that commercial Ready-to-Use Foods and lipid-based supplements were any better than flour porridge made locally from enriched blended food for the treatment of severe and moderate acute malnutrition. Ready-to-Use products are often used in appeals (see right).
While they can be convenient their use must be limited and carefully managed. We are calling for robust evidence of efficacy and impact on traditional foods before products are promoted.
● See pg 17, pg 31, Update 45 and IBFAN statement.
Action in Portugal, Ireland and Greece
New IBFAN groups, in Greece and Portugal are drawing attention to Code violations. Jacqueline de Montaigne prompted Portugal's National Health Board (DGS) to coordinate a national breastfeeding campaign. She says this is not a 'mummy war of breastfeeding vs. formula feeding.. we are not trying to entice mothers to breastfeed but fighting for the right for all mothers to choose how they feed their babies and that this choice be based on accurate scientific evidence, free from commercial influence.'
Baby Feeding Law Group Ireland (BFLGI) is a new alliance of 20 organisations and professional groups who provide services to families and young children and wish to strengthen the Irish law, protect the health of all babies in Ireland and ending harmful marketing practices. Ethical Sponsorship Ireland's Facebook page highlights Code violations and raises awareness.
● Contact: Claire Allcutt: BFLGIreland@gmail.com
UNICEF, SUN and Conflicts of Interest
IBFAN has been criticising the Scaling Up Nutrition (SUN) initiative for many things, including its lack of attention to conflicts of interest. SUN’s governing body includes major corporations and a key factor of SUN’s approach is the establishment of Public Private Partnerships in developing countries - with businesses at their core.
In response to our criticisms, SUN is now attempting to produce Conflicts of Interest (COI) guidance and on its website describes the process being managed by the Global Social Observatory (GSO). GSO is a body that is not independent of commercial interests and its efforts so far are disappointing. In particular they leave out any guidance for SUN’s own governance - ignoring the WHA 65.6 Resolution (2012), which requires COI to be addressed at all levels, not just by Member States at country level.
GSO’s ‘Reference Note’ shows poor understanding of the COI concept and the existence of irreconcilable conflicts of interest. It mixes COI with ‘Conflict resolution’ and uses concepts such as ‘mutual accountability’ that assign roles to governments that may not be democratic. No reference is available for statements such as ‘there is more to be gained by engaging all groups that are working to improve nutrition.’
● Meanwhile we are pleased that SUN has changed the reference to breastfeeding on its website. Its strategy 2012-15 still refers only to exclusive breastfeeding for 6 months, but its website refers to ‘Support for exclusive breastfeeding up to 6 months of age and continued breastfeeding together with appropriate and nutritious food up to 2 years of age, fortification of foods, micro-nutrient supplementation, treatment of severe malnutrition.’
Doctors call to end sponsorship
The International Society for Social Pediatrics and Child Health (ISSOP) has adopted a strong Position Statement explaining why baby feeding industry sponsorship damages health and the reputation of paediatricians. http://issop.org
 UNICEF's Landscape Analysis
UNICEF's Landscape Analysis

UNICEF published a new paper in September entitled Breastfeeding on the Worldwide Agenda - a landscape analysis on political commitment for programmes to protect, promote and support breastfeeding. The report is based on interviews, including with IBFAN. While the report rightly identifies breastfeeding as a seriously overlooked issue, we are concerned about many of its observations and conclusions. Its focus on the promotion of breastfeeding (rather than its protection) and its failure to stress the obligations of governments to protect child rights. While identifying relations with the private sector as an area needing further consultation, the report suggests that private sector involvement and public private partnerships are an essential component in the planning and implementation of child survival initiatives. We see this approach, also used by SUN, as being highly problematic, and overlooking the Global Strategy on Infant and Young Child Feeding that clearly identifies two appropriate roles for the Private Sector: make safe products and adhere to the International Code.
UNICEF shares our understanding that marketing practices that violate the International Code are a key obstacle to ensuring that every mother can make an informed decision about how to feed her child and we will be discussing with them modes of collaboration that would advance implementation of the Global Strategy and other relevant WHA policies.






